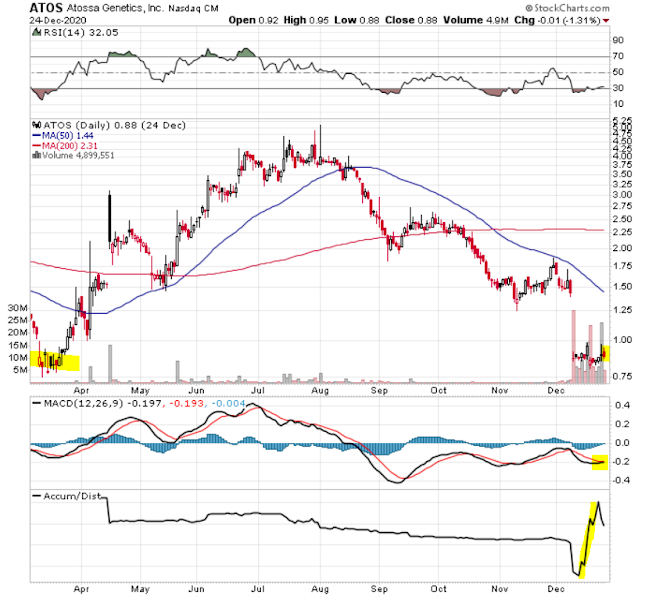
28.DEC.2020 DD ATOS (Attossa Therapeutics)
Website: Atossa Therapeutics
Free Float: 25M
Atossa Announces $20.0 M Underwritten Public Offering
Atossa Announces $14.0 M Registered Direct Offering Priced At-The-Market
2. Commence Phase 2 study in Sweden for our Endoxifen to reduce MBD
3. Phase 2 for breast density/mammography adjuvant planned for Q1 2021
Atossa has 2 programs to develop therapies to treat COVID-19:
1. AT-H201 for severely ill patients to improve lung function and reduce the amount of time that COVID-19 patients are on ventilators.
Launched in April 2020.
The program uses a novel combination of two drugs that have been previously approved by the FDA for other diseases.
2. AT-301 Nasal Spray for at-home use immediately following diagnosis of COVID-19 to proactively reduce symptoms of COVID-19 and to slow the infection rate so that a person’s immune system can more effectively fight the virus.
Atossa also intends to conduct testing to determine whether AT-301 can be used as a prophylaxis to prevent or mitigate SARS-CoV-2, with the goal that it could become a “bridge to the vaccine” and be useful in the next phase of the coronavirus pandemic.









Comments
Post a Comment